Sepsis: a long-term battle, new issues and goals
This article was originally published in L'Édition n°25.
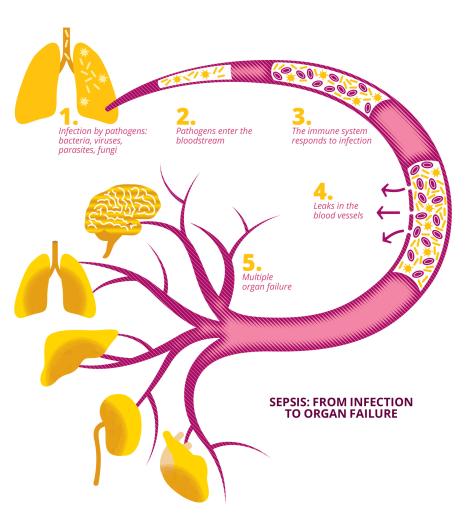
Sepsis (or septicaemia) is a disease resulting from a dysregulated immune system response to infection. It affects several million people a year, including children, and is one of the world's leading causes of death, now representing a major public health problem. How can this disease be effectively treated? What solutions can be offered to patients? How can complications be avoided? These are questions that the Prometheus University Hospital Institute (IHU), which comprises Université Paris-Saclay, the French Alternative Energies and Atomic Energy Commission (CEA), Greater Paris University Hospitals (AP-HP), the National Institute of Health and Medical Research (Inserm) and other academic, associative and industrial partners, aims to answer. Officially launched on 17 September 2024, this first centre in the world to integrate prevention, treatment, research, education and technology transfer within a single ecosystem, aims to halve mortality and sequelae caused by sepsis within ten years.
Defined by the World Health Organization (WHO) as one of the most frequent causes of death worldwide, sepsis (or septicaemia) is a serious condition resulting from a loss of control of the inflammatory system following an infection, leading to impairment of the body's vital functions. According to the UN organisation, it affects almost 50 million people around the world every year (45% of them children under the age of five), and is responsible for one in five deaths. It also causes mental and motor disabilities in half of all survivors.
"Sepsis is a dysimmune disease caused by an initial bacterial, viral, parasitic or fungal infection with an acute and sudden onset. The disease is also defined by its long-term evolutionary potential, with relapses experienced by around a third of first-time patients, and after-effects impacting cognitive functions and leading to physical disabilities or the triggering of new inflammatory or autoimmune diseases," describes Djillali Annane, head of the neuroendocrine response to sepsis study team (SEPSIS), within the Infection and Inflammation Laboratory (2I - Univ. Paris-Saclay/UVSQ/Inserm). A specialist in this disease, to which he has devoted thirty years, Djillali Annane is now head of the Prometheus University Hospital Institute (IHU), whose aim is to halve the mortality rate and the social and financial cost of sepsis within the next ten years. In 2019 alone, there were over 400 cases of sepsis per 100,000 inhabitants in France, with hospitalisation costs of around €16,000 per person.
A new approach to sepsis
The 2023 winner of the call for projects issued as part of the government's France 2030 Plan, the Prometheus IHU brings together more than 250 scientists from some 60 laboratories, mainly from Université Paris-Saclay, the French Alternative Energies and Atomic Energy Commission (CEA), Greater Paris University Hospitals (AP-HP), and the National Institute of Health and Medical Research (Inserm), as well as their academic, associative and industrial partners, to fight this disease and the health, social and economic burden it represents. Integrating researchers, caregivers, patients, private partners and institutions within an ecosystem of prevention, care, research, education and technology transfer, it should help develop new diagnostic tests and new drugs.
In addition to research teams that already specialise in sepsis and have worked on this disease for many years, the Prometheus IHU also involves new teams whose subject matter is more remote, with the aim of uncovering disruptive innovations. "We are bringing together colleagues whose areas of expertise include mathematics, chemistry, physics, climate, fundamental immunology, veterinary science, etc. and who will be devoting part of their research time over the next ten years to sepsis, leading original, diverse and unprejudiced approaches," enthuses Djillali Annane. The stakes are high, as despite decades of extensive research, the mechanisms behind the disruption of the immune response to pathogens are still poorly understood, and there is still no entirely satisfactory treatment. One of the scientific ambitions of the Prometheus IHU is to gain a better understanding of the molecular and cellular interactions between the host organism and the pathogens involved in the progression from uncomplicated infection to sepsis. A longitudinal cohort of 10,000 patients, monitored over ten years, should make it easier to understand the social and economic consequences of long-term sepsis.
Corticoids: a first step towards curbing sepsis mortality
Generally speaking, when an organism is confronted with a pathogen (bacterium, virus, parasite, fungus, etc.), its immune system triggers an adapted response that circumscribes the pathogen's development before destroying it and removing it from the body. "Most infections are generally pretty harmless to the body," says Djillali Annane. "In the vast majority of cases, infections are only moderately symptomatic, and many disappear thanks to our natural defences alone."
In some cases, however, the immune response to an infection in the body is disproportionate, in that it is either excessive, damaging a large number of tissues and impairing the functioning of the body's organs, or it is insufficient, exposing the body to the risk of superinfection. "We speak of immunoparalysis when the immune system becomes fragile and is unable to respond to any new aggression," explains the professor. In their search for a treatment for sepsis, from 1994, Djillali Annane and his team demonstrated the benefits of using corticoid-based treatment with moderate time and dosage scales, to reduce the mortality associated with the disease. Corticoids are a group of steroid hormones secreted by the body's adrenal glands, located in the abdomen. They influence stress, sugar use and the body's immune and inflammatory systems. "Corticoids are part of the body's defence arsenal; cortisol, aldosterone, etc. are naturally produced by our bodies to defend themselves against external aggression, including pathogens and infections," confirms Djillali Annane. Corticoids also exist today in the form of synthetic molecules, which have been developed since the 1950s.
Used in high concentrations, corticoids have an immunosuppressive effect that disrupts the activity of the immune system. "We are working on the contribution of corticoids to this dysregulation, this dysimmune response to infection synonymous with sepsis. We have shown that, through a variety of mechanisms, pathogens do indeed cause an alteration in the defence system, including corticoids, and contribute to a disproportionate response to the threat by the body. Fortunately, corticoids can now be synthesised. We therefore conducted experiments aimed at using corticoids to compensate for this deregulation of the body. We have done a lot of work on the molecular mechanisms involved, on animal and cell models, and also on patients, to try and better understand the different stages that alter the body's defence mechanisms and how to compensate for them," explains Djillali Annane, a true pioneer in the treatment of the disease through the controlled administration of corticoids. "We were the first to establish that a certain modality in administering the combination of hydrocortisone with fludrocortisone, given in moderate doses over durations not exceeding seven days, compensates, at least partially, for the alteration that caused sepsis," confirms the researcher.
The need for precision medicine
However, as each human's sensitivity to treatments and molecules is highly variable, Djillali Annane and his team have spent ten years identifying patient "profiles". "In medicine, we know that it is possible to improve the efficiency of treatments by personalising their administration. This is achieved by identifying those patients who will particularly benefit from a treatment, and those who, on the contrary and for various reasons, will receive no benefit - or worse, whose situation will deteriorate. This is precision medicine."
For this, they use molecular biology tools such as transcriptomics (the study of ribonucleic acids, RNA) and metabolomics (the study of metabolites), combined with predictive artificial intelligence tools such as machine learning and deep learning. "We are trying to define signatures, prints that we can then use at a patient's bedside to determine whether they will benefit from corticoid treatment. In a way, our work on corticoid therapy represents a ‘proof of concept’. Now that this has been established, the Prometheus IHU aims to roll it out and embrace the different possible therapeutic options, particularly in terms of immunotherapy," says the doctor. "Ultimately, this ‘profiling’ will be beneficial beyond sepsis, for example in cases of inflammatory disease or cancer." Djillali Annane's team will be working with other international teams to check that this profiling can be adapted to any socio-economic context.
Precision medicine is also at the heart of Prometheus IHU's scientific ambitions, with a particular focus on validating and marketing a rapid testing platform to characterise host response to infection and therapeutic targets at the individual level. This will involve creating a sepsis-specific digital twin to accurately anticipate the individual's response to treatment.
Bacteria and childhood sepsis
Director of Research in the Pathogen, Immunity, Microbiota (PIMs) team at the Food Microbiology for Health Laboratory (MICALIS - Univ. Paris-Saclay/French National Research Institute for Agriculture, Food and Environment, INRAE), Nalini Rama Rao has been studying the interactions between pathogenic bacteria and the host immune response for over twenty years. In particular, she has deciphered the virulence mechanisms of the Bacillus cereus bacterium. "It is a known food contaminant, but I have been studying it more from the angles of hospitals and virulence factors: Bacillus cereus is responsible for serious infections in newborns, particularly septicaemia," explains the researcher. "Hospitals were not really concerned about this bacterium for a long time, as it was a contaminant of bedding and carers. I wanted to show that it was a true pathogenic bacterium, with a real impact on this target group," says Nalini Rama Rao, who is particularly keen to ensure that her research has clinical relevance. "For example, I have worked with hospitals to change the procedures linked to collecting breast milk, and the feeding tubes for premature infants, to reduce the presence of Bacillus cereus and thus the risk of septicaemia," she explains.
Sepsis is the third leading cause of infant mortality worldwide and is responsible for the death of over 300,000 newborns every year. Bacterial resistance to antibiotics complicates the treatment of infections and increases the risk of sepsis. Nalini Rama Rao therefore extended her research beyond Bacillus cereus and is currently studying how to block the virulence of pathogenic bacteria that are resistant to current treatments and cause sepsis.
When antibiotic resistance adds to the problem
But how can we fight pathogens effectively today, when their survival in the face of the antibiotics designed to destroy them (antibiotic resistance) is increasing worldwide? "Current antibiotics work by targeting general bacterial survival mechanisms. Basically, the antibiotic will inhibit the bacterium's ability to survive, whatever the circumstances," explains Nalini Rama Rao. "It is effective, but it has a twofold deleterious effect, in that it impacts all the bacteria in the host's microbiota, whose importance we now know, and causes collateral health damage. The other problem is resistance induction. Bacteria mutate to survive, becoming increasingly resistant to the antibiotics originally designed to eliminate them." New therapeutic solutions are therefore needed that do not contribute to antibiotic resistance.
In 2017, the WHO published for the first time a list of pathogens for which antibiotic resistance is becoming critical. In May 2024, three new bacterial families were added to this list. Six of these most antibiotic-resistant pathogens - Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and the Enterobacter species - form the acronym ESKAPE. All these bacteria, and Bacillus cereus in particular, share a common protein, namely the Mfd (mutation frequency decline) protein. "This protein, which I initially identified as a virulence factor in B. Cereus, is produced by all bacteria and is essential for them to resist the host immune system," explains Nalini Rama Rao. Following this discovery, the researcher studied the possibility of targeting Mfd in the development of new drugs, while taking a sensitive approach to antibiotic resistance. "Rather than kill the bacteria, the idea is to disarm them. Mfd is a protein that is only useful at times of immune stress and inflammation. We are developing a compound that blocks the protein at that precise moment, making the bacteria vulnerable to the immune system, which then gets rid of it. As the microbiota is not a target of the immune system, our compound has an impact on the pathogenic bacteria, without affecting the microbiota."
A universal protein that paves the way
For Nalini Rama Rao, studies linked to this protein open up new perspectives: "After all, this protein is universal; it is conserved by all bacteria. That is where I got the idea to extend our knowledge to other pathogens and bacteria responsible for antibiotic resistance and sepsis." Following this real proof of concept, Nalini Rama Rao and her team have seen their project financed by the AXA Mutual Health Fund to the tune of around one million euros, with the aim of developing a preclinical research project and ultimately creating new drugs. The team has been awarded funding from the French National Research Agency (ANR), to continue its fundamental research on the subject. The project is also supported by Université Paris-Saclay, via the POC in Labs maturation programme and the SATT Paris-Saclay, the Technology Transfer Acceleration Office of the Paris-Saclay Cluster.
The development of new treatments, such as innovative small molecules, nanomedicines, biotherapies and vaccines, as well as strategies to modulate microbiota, is also one of Prometheus IHU's key research areas.
Nalini Rama Rao is convinced that the fight against antibiotic resistance is a constant battle that everyone can join, and is developing related projects that go far beyond research. In 2019, the researcher joined forces with cartoonist Claire Fouquet to publish a comic strip, Résistances croisées (Cross-resistances), which describes life in the laboratory and the PIMs team, presenting the major challenges of antibiotic resistance. Three years later, a new, larger-scale project was born - a cooperative board game, Propag'action, based on the use of cards and suitable from the age of ten.
Publications :
- Pitre, T., et al. Corticosteroids in community-acquired bacterial pneumonia: a systematic review, pairwise and dose-response meta-analysis. Journal of General Internal Medicine, 38(11), 2023.
- Tran, SL. et al. An anti-virulence drug targeting the evolvability protein Mfd protects against infections with antimicrobial resistant ESKAPE pathogens. BioRxiv, 2024
- https://bd-bacterie.com/

This article was originally published in L'Édition n°25.
Find out more about the journal in digital version here.
For more articles and topics, subscribe to L'Édition and receive future issues: