Catalysis: a tool for green chemistry
(This article was originally published in L'Édition N.23)
By using a substance (called a catalyst) that undergoes little or as little as possible transformation, catalysis opens the way to accelerating and even selectivising chemical reactions of all kinds. Catalysis has been observed for thousands of years and is now one of the pillars of sustainable chemistry that consumes fewer resources and less energy. Overview of the practices linked to this approach at Université Paris-Saclay.
Catalysis is used during a chemical reaction to accelerate or promote a particular synthesis pathway of the reaction. Using a catalyst, it is therefore possible to consume fewer chemical reagents or obtain greater quantities of a chemical of interest. The second advantage of catalysis generally lies in the reuse of the catalyst after the reaction, as it is not consumed. In this sense, catalysis and its many avenues are a real pillar of sustainable, environmentally-friendly chemistry. On 23 November 2023, chemists from Université Paris-Saclay met for the annual Scientific Day of Université's Chemistry Graduate School (GS), focusing on the challenges of sustainable chemistry and the future of catalytic chemistry.
Humans have been observing all types of catalysis for thousands of years. However, it was not until 1835 that Swedish chemist Jöns Jacob Berzelius first coined the term catalysis. In his annual report the same year, the chemist wrote: "It is then shown that several simple and compound bodies, soluble and insoluble, have the property of exercising on other bodies an action very different from chemical affinity. The body effecting the changes does not take part in the reaction and remains unaltered through the reaction. This unknown body acts by means of an internal force, whose nature is unknown to us." Almost two centuries after this first definition, catalysis has become a mainstay of modern chemistry, and there many different types of catalysis and just as many new disciplines, such as catalysis using organic molecules or metallic elements, photocatalysis (induced by light) and biocatalysis (inspired by natural phenomena).
Exploring the living world
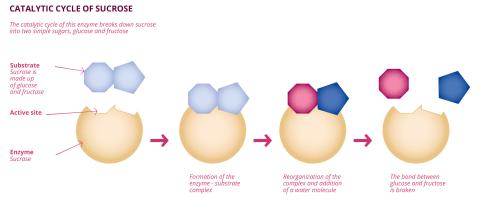
Carine Vergne and Anne Zaparucha are members of the Genomics Metabolics joint research unit (GM - Univ. Paris-Saclay, Univ. Évry, CNRS, CEA). For over 15 years, the two scientists have been studying a multitude of chemical reactions and seeking to develop new biocatalytic pathways for the synthesis of these reactions, using enzymes, the catalytic proteins found in nature. "Biocatalysis is the use of enzymes, commonly called living catalysts, in organic chemistry," explains Anne Zaparucha. “Living organisms are universally subject to a series of successive reactions known as metabolism. Each of these reactions is catalysed by a corresponding enzyme." Through promiscuity, the enzyme corresponding to a specific metabolic reaction in a living organism can be used in the synthesis of another compound, provided that the synthesis process and the obtained product are similar to the metabolic reaction. "Biocatalysis is based on this property of promiscuity. An enzyme reacts with its metabolic substrate, as well as with other substrates that are generally close to it," explains Anne Zaparucha. It is this property of enzyme promiscuity that makes biocatalysis possible. "What's more, we now have an absolutely extraordinary range of tools for modifying enzymes at will." In 2018, American researcher Frances Arnold was awarded the Nobel Prize in Chemistry for her work on the directed evolution of enzymes.
Carine Vergne and Anne Zaparucha have been investigating since 2008, looking for activities, combinations of chemical reactions and enzymes of interest. "In concrete terms, we focus on a particular reaction and then look for the enzymes capable of catalysing that reaction. We also prefer to target reactions for which finding a biocatalytic alternative is of interest, either economically because the reaction is widely used or, on the contrary, because the targeted reaction is not widely used due to the complexity of its execution," explains Carine Vergne. The two researchers are interested in a wide range of reactions, including oxidation, halogenation and hydroxylation, although reductive amination is by far the best known. "We generally begin our work with a bibliography, in particular by reading up on the elucidation of biosynthesis pathways. Our task is then to find the ideal enzymes to implement in these reactions. It is not the only reaction we are working on, but reductive amination remains one of our laboratory's flagship projects, not least due to the number of publications on the subject. It is a very popular reaction," explains Carine Vergne. "This is a very important reaction in chemistry. Its field of application is quite extraordinary since two-thirds of therapeutic ally active compounds today incorporate an amine in their structure," adds Anne Zaparucha. Reductive amination results from the addition of an amine group (notably composed of a nitrogen atom) to an organic molecule.
Bringing diversity
As the laboratory's activities have progressed, the two researchers and their colleagues have helped to build up a veritable library of enzymes and their variations. This collection is for their use, but will also benefit the rest of the biocatalysis community. "Compared with other teams researching other biocatalysts, we try to choose activities in which the enzymes used are rarely studied or their variations little known. What is different about us is our desire to bring diversity to the established bibliographical knowledge of enzymes. What we are seeing in biocatalysis is a potential lack of diversity in the available enzyme portfolios," explains Carine Vergne. "It is this exploration of biodiversity that is our strength," says Anne Zaparucha. "It is essential to our discipline. Unlike conventional chemical catalysis, where a catalyst often has fairly wide ′coverage′, an enzyme is active on a handful of products at most. So, to cover a wide range of substrates, we need a wide range of enzymes. That is our strength; we prefer to discover new enzymes rather than modify existing ones. And each time, it brings diversity to the community."
The GM JRU is part of Genoscope, the French National Sequencing Centre. The explorations of Anne Zaparucha, Carine Vergne and their colleagues are made possible by the genomic sequencing laboratory and specialist bioinformatics teams. "We use this partnership with Genoscope and the bioinformatics expertise of these teams to carry out our research. We start with a known enzyme sequence listed in the bibliography to catalyse the reaction we are interested in. Thanks to Genoscope, we can search the biodiversity for homologous sequences that meet more or less similar criteria. The aim is then to produce these sequences and test them within our initially chosen reaction, like ′traditional′ chemists. This is followed by a phase in which we analyse the results and products obtained, and a phase of optimisation of the reaction conditions," explains Carine Vergne.
Photocatalysis, porphyrins and MOFs: an innovative route to formate synthesis
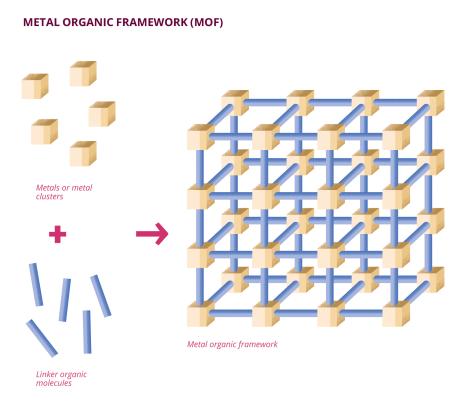
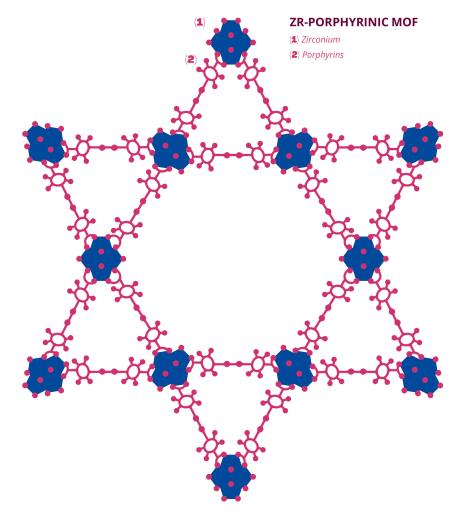
At the Lavoisier Institute of Versailles (ILV - Univ. Paris-Saclay, UVSQ, CNRS), Anne Dolbecq and Pierre Mialane study the reduction of carbon dioxide (CO2) to formate (HCO2 -), a widely used chemical precursor. The two scientists carry out this chemical reaction via the photocatalysis of a porphyrin metal-organic network. Porphyrin is a molecule composed of rings of several carbon atoms and one nitrogen atom. Metal-organic frameworks (MOFs) are macromolecules composed of organic ligands (mainly carbon) and metal clusters or ions. Their porous structure makes these molecules particularly interesting for catalytic chemistry. In addition, some MOFs can "capture" smaller molecules, such as carbon dioxide. In this case, the porphyrinic MOF studied by Anne Dolbecq and Pierre Mialane contains zirconium, a metal to which carbon dioxide "binds" within the structure. The carbon dioxide is then reduced to formate thanks to a specific chemical species, triethanolamine (TEOA), and light rays. "Porphyrin is irradiated with light, which places it in an excited state, meaning that it has more energy than it needs," explains Pierre Mialane. "Once excited, porphyrin and TEOA create a sacrificial electron-donor radical species, providing an electron and leading to the reduction of carbon dioxide to formate." This reaction is described as photocatalytic, since it is irradiation by artificial light rays that triggers the complex process leading to formate synthesis. "We use the photosensitive nature of the porphyrin in our MOF to trigger electron transfer and thus generate the reduction of carbon dioxide to formate. Porphyrins have long been known to be photosensitive species, so they act as antennae in our MOF," explains Anne Dolbecq. Once the reaction is complete, the MOF can be reused an average of three times.
Anne Dolbecq and Pierre Mialane have been studying the MOF composed of porphyrin and zirconium for the last five years. ILV has been a veritable bastion of MOFs, especially since their discovery in the 1990s. "One family of MOFs, the MILs (Lavoisier Institute Materials), originated in the laboratory," say the two scientists. In this case, the zirconium porphyrin MOF studied by the two chemists was not designed in the ILV laboratories; Anne Dolbecq and Pierre Mialane discovered it by chance and studied the mechanisms involved in the reduction of carbon dioxide using this compound. "We were not the first to synthesise this MOF; it already existed in the literature. As did the photosensitivity of porphyrin. However, the reduction mechanisms and the role of the TEOA were very unclear," recalls Anne Dolbecq.
The two scientists were originally specialists in polyoxometalate (POM), compounds that lead to the oxidation of water, for example. Today, their new expertise in MOFs and carbon dioxide reduction by MOF and photocatalysis is opening up new avenues. "Imagine combining a POM that oxidises water and supplies electrons to reduce carbon dioxide with our MOF. This would lead to the synthesis of formate by reducing carbon dioxide using water molecules. More TEOA, and a 'prettier', cleaner mechanism!" comments Pierre Mialane. "In the future, we could imagine placing devices on factory chimneys that release carbon dioxide and water vapour to form formate," adds Anne Dolbecq.
How to drive a greener catalytic converter
Of all the existing catalysis techniques, the most popular is undoubtedly metalorganic catalysis, which involves a complex composed of an organic structure and a metal. Palladium catalysis is particularly popular and is the subject of research by Japanese researchers Ei-ichi Negishi and Akira Suzuki and American researcher Richard Heck, all three of whom were awarded the 2010 Nobel Prize in Chemistry. While the effectiveness of palladium as a catalyst has been proven, its widespread use has been the subject of debate. "In practical terms, it is a costly element whose global reserves are beginning to dwindle," says Christine Tran, senior lecturer and researcher in the Biomolecules: Design, Isolation, Synthesis Laboratory (BioCIS - Univ. Paris-Saclay, CNRS, CY Cergy Université). "Today, palladium and other metal catalysts are very widely used, particularly in the pharmaceutical industry. The subject of an alternative to these metals is very topical. As chemists, our role today is to find new, more eco-friendly catalysts. For example, I am currently developing a research theme focusing on photocatalysis, a cleaner form of catalysis than that based on the use of heavy metals, but also one that is easier to access; with light as the catalyst, there is no need for delicate handling, cumbersome tools such as glove boxes." adds the chemist.
At BioCIS, Christine Tran aims to develop new eco-friendly strategies in medicinal chemistry, in particular for the synthesis of compounds such as indoles, which inhibit the development of leukaemia in mice, and dihydrobenzocarbazoles, which are anti-parasitic molecules. "At my level, eco-design starts with the choice of solvent used for synthesis," she explains. "I also think about how I am going to extract my final product. Using a silica column to purify a product is best avoided, given the toxicity of silica, in favour of recrystallisation, for example."
References
- Ducrot L. et al, Expanding the Substrate Scope of Native Amine Dehydrogenases through In Silico Structural Exploration and Targeted Protein Engineering, ChemCatChem, Vol. 14, Issue 22, 2022.
- Benseghir Y. et al, Unveiling the mechanism of the photocatalytic reduction of CO2 to formate promoted by porphyrinic Zr-based metal–organic frameworks, J. Mater. Chem. A, 2022.
- Tran C. et al., Iron-catalyzed reductive cyclization of nitroarenes: Synthesis of aza-heterocycles and DFT calculations, Chinese Chemical Letters, Volume 34, Issue 3, 2023.
This article was originally published in L'Edition No. 23.
Find out more about the journal in digital version here.
Subscribe to the newsletter and never miss an issue.